Difference between revisions of "Atom"
| Line 1: | Line 1: | ||
[[Image:lighterstill.jpg]] | [[Image:lighterstill.jpg]] | ||
| − | [[Image:Helium_atom_QM.svg.png|right]] | + | [[Image:Helium_atom_QM.svg.png|right|thumb|This illustrates the [[atomic nucleus|nucleus]] (pink) and the [[electron cloud]] distribution (black) of the [[Helium]] atom. The nucleus (upper right) is in reality spherically symmetric, although this is not always the case for more complicated nuclei.]] |
| + | |||
In [[chemistry]] and [[physics]], an '''atom''' ([[Greek language|Greek]] ''ἄτομος'' or ''átomos'' meaning "indivisible") is the smallest particle still characterizing a [[chemical element]]."Atom" in IUPAC Compendium of Chemical Terminology, Electronic version, [http://goldbook.iupac.org/A00493.html]. | In [[chemistry]] and [[physics]], an '''atom''' ([[Greek language|Greek]] ''ἄτομος'' or ''átomos'' meaning "indivisible") is the smallest particle still characterizing a [[chemical element]]."Atom" in IUPAC Compendium of Chemical Terminology, Electronic version, [http://goldbook.iupac.org/A00493.html]. | ||
| Line 18: | Line 19: | ||
The father of modern atomic theory was a Jesuit priest, [[Ruggero Boscovich]], who based his theory mostly on [[classical mechanics]] ([[Isaac Newton|Newtonian]] mechanics) and published it in [[1758]]. The theory was further developed by [[Amedeo Avogadro]], his brother Johann Avogadro and the developers of the [[kinetic theory of gases]] such as [[James Clerk Maxwell]] and physicist [[Ludwig Boltzmann]]. Boscovich was regarded as the father of modern atomic theory by Faraday, Mendeleev, Maxwell, and Kelvin, who observed that his and the work of others' were "all developments of Boscovich's theory pure and simple." | The father of modern atomic theory was a Jesuit priest, [[Ruggero Boscovich]], who based his theory mostly on [[classical mechanics]] ([[Isaac Newton|Newtonian]] mechanics) and published it in [[1758]]. The theory was further developed by [[Amedeo Avogadro]], his brother Johann Avogadro and the developers of the [[kinetic theory of gases]] such as [[James Clerk Maxwell]] and physicist [[Ludwig Boltzmann]]. Boscovich was regarded as the father of modern atomic theory by Faraday, Mendeleev, Maxwell, and Kelvin, who observed that his and the work of others' were "all developments of Boscovich's theory pure and simple." | ||
| − | |||
In 1803, [[John Dalton]] used the concept of atoms to explain why elements always reacted in [[Law of multiple proportions|simple proportions]], and why certain gases dissolved better in water than others. He proposed that each element consists of atoms of a single, unique type, and that these atoms could join to each other, to form compound chemicals. | In 1803, [[John Dalton]] used the concept of atoms to explain why elements always reacted in [[Law of multiple proportions|simple proportions]], and why certain gases dissolved better in water than others. He proposed that each element consists of atoms of a single, unique type, and that these atoms could join to each other, to form compound chemicals. | ||
Revision as of 00:52, 11 January 2008
In chemistry and physics, an atom (Greek ἄτομος or átomos meaning "indivisible") is the smallest particle still characterizing a chemical element."Atom" in IUPAC Compendium of Chemical Terminology, Electronic version, [1].
An atom consists of a dense atomic nucleus of positively-charged protons and electrically-neutral neutrons, surrounded by a much larger electron cloud consisting of negatively-charged electron. An atom is electrically neutral if it has the same number of protons as electrons. The number of protons in an atom defines the chemical element to which it belongs, while the number of neutrons determines the isotope of the element.
History
The idea that matter is composed of discrete units and can not be divided into any arbitrarily small quantities has been around for thousands Of years. The earliest references to the concept of atoms date back to ancient India in the 6th century BCE. <ref>Gangopadhyaya, Mrinalkanti. Indian Atomism: History and Sources. Atlantic Highlands, New Jersey: Humanities Press, 1981. ISBN 0-391-02177-X. The Nyaya and Vaisheshika schools developed elaborate theories on how atoms combined into more complex objects (first in pairs, then trios of pairs) [2]. The references to atoms in West, emerge a century later by Leucippus whose student, Democritus, sytemized his views. In around 450 BCE, Democritus coined the term atomos, which meant "uncuttable". Though both the Indian and Greek concepts of the atom were based purely on philosophy, modern science has retained the name coined by Democritus.
The father of modern atomic theory was a Jesuit priest, Ruggero Boscovich, who based his theory mostly on classical mechanics (Newtonian mechanics) and published it in 1758. The theory was further developed by Amedeo Avogadro, his brother Johann Avogadro and the developers of the kinetic theory of gases such as James Clerk Maxwell and physicist Ludwig Boltzmann. Boscovich was regarded as the father of modern atomic theory by Faraday, Mendeleev, Maxwell, and Kelvin, who observed that his and the work of others' were "all developments of Boscovich's theory pure and simple."
In 1803, John Dalton used the concept of atoms to explain why elements always reacted in simple proportions, and why certain gases dissolved better in water than others. He proposed that each element consists of atoms of a single, unique type, and that these atoms could join to each other, to form compound chemicals.
In 1897, JJ Thomson, through his work on cathode rays, discovered the electron and its subatomic nature (i.e., its lightness compared with the mass of atoms), which destroyed the concept of atoms as being indivisible units. Later, Thomson also discovered the existence of isotopes through his work on ionized gases.
Thomson believed that the electrons were distributed evenly throughout the atom, balanced by the presence of a uniform sea of positive charge. However, in 1909, the gold foil experiment was interpreted by Ernest Rutherford as suggesting that the positive charge of an atom and most of its mass was concentrated in a nucleus at the center of the atom (Rutherford model), with the electrons orbiting it like planets around a sun. In 1913, Niels Bohr added quantum mechanics into this model, which now stated that the electrons were locked or confined into clearly defined orbits, and could jump between these, but could not freely spiral inward or outward in intermediate states.
In 1926, Erwin Schrodinger, using Louis DeBroglie's 1924 proposal that all particles behave to an extent like waves, developed a mathematical model of the atom that described the electrons as three-dimensional waveforms, rather than point particles. A consequence of using waveforms to describe electrons, pointed out by Werner Heisenberg a year later, is that it is mathematically impossible to obtain precise values for both the position and momentum of a particle at any point in time; this became known as the uncertainty principle. In this concept, for any given value of position one could only obtain a range of probable values for momentum, and vice versa. Although this model was difficult to visually conceptualize, it was able to explain many observations of atomic behavior that previous models could not, such as certain structural and spectral patterns of atoms bigger than hydrogen. Thus, the planetary model of the atom was discarded in favor of one that described orbital zones around the nucleus where a given electron is most likely to exist.
Subatomic particles
Though the word atom originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the "atom" is composed of various subatomic particles, including:
- electrons, which have a negative charge, a size which is so small as to be currently unmeasurable, and which are the least heavy (i.e., massive) of the three types of basic particles, with a mass of 9.11x10-31kg.
- protons, which have a positive charge, with a free mass about 1836 times more than electrons (mass of 1.67x10-27kg though binding energy changes can reduce this).
- neutrons, which have no charge, have a free mass about 1839 times the mass of electrons, and about the same physical size as protons (which is on the order of 2.5x10-15 m in diameter, although the "surface" of a proton or neutron is not very sharply defined).
Protons and neutrons make up a dense, massive atomic nucleus, and are collectively called nucleons. The electrons form the much larger electron cloud surrounding the nucleus. Both protons and neutrons are themselves now thought to be composed of even more elementary particles, quarks.
Atoms of the same element have the same number of protons (called the atomic number). Within a single element, the number of neutrons may vary, determining the isotope of that element. The number of electrons associated with an atom is most easily changed, due to the lower energy of binding of electrons. The number of protons (and neutrons) in the atomic nucleus may also change, via nuclear fusion, nuclear fission, bombardment by high energy subatomic particles or photons, or certain (but not all) types of radioactive decay. In such processes which change the number of protons in a nucleus, the atom becomes an atom of a different chemical element.
Atoms are electrically neutral if they have an equal number of protons and electrons. Atoms which have either a deficit or a surplus of electrons are called ions. Electrons that are furthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism atoms are able to bond into molecules and other types of chemical compounds like ionic and covalent network crystals.
Atoms and molecules
For gases and certain molecular liquids and solids (such as water and sugar), molecules are the smallest division of matter which retains chemical properties; however, there are also many solids and liquids which are made of atoms, but do not contain discrete molecules (such as salts, rocks, and liquid and solid metals). Thus, while molecules are common on Venus (making up all of the atmosphere and most of the oceans), most of the mass of the Earth (much of the crust, and all of the mantle and core) is not made of identifiable molecules, but rather represents atomic matter in other networked arrangements, all of which lack the particular type of small-scale interrupted order (i.e., small, strongly-bound collections of atoms held to other collections of atoms by much weaker forces) that is associated with molecular matter.
Most molecules are made up of multiple atoms; for example, a molecule of water is a combination of two hydrogen atoms and one oxygen atom. The term "molecule" in gases has been used as a synonym for the fundamental particles of the gas, whatever their structure. This definition results in a few types of gases (for example inert elements that do not form compounds, such as neon), which has "molecules" consisting of only a single atom.
Big Bang Theory
According to the Big Bang theory, immediately after the start of the Big Bang, space expanded incredibly quickly in a very short time. This process is called inflation. After that, energy density got lower because of inflation/expansion and different kinds of subatomic particles including quarks and electrons made their appearance. Just one millionth of a second after the birth of the universe, the quarks had clumped together to form new particles called protons and neutrons. After a hundred seconds or so, some of the protons and nearly all of the neutrons gathered into bunches, consisting of two protons and two neutrons. Eventually, each bunch, or atomic nucleus, captured two electrons to form a helium atom, and each remaining proton captured a single electron to form a hydrogen atom. The first building blocks of matter had been born.
In models of the Big Bang, Big Bang nucleosynthesis predicts that within one to three minutes of the Big Bang almost all atomic material in the universe was created. During this process, nuclei of hydrogen and helium formed abundantly, but almost no elements heavier than lithium. Hydrogen makes up approximately 92% of the atoms in the universe (by number, not mass); helium makes up less than 7%; and all other elements make up less than 1% (see Abundance of the chemical elements). However, although nuclei (fully-ionized atoms) were created, neutral atoms themselves could not form in the intense heat.
Big Bang chronology of the atom continues to approximately 380,000 years after the Big Bang when the cosmic temperature had dropped to just 3,000 K. It was then cool enough to allow the nuclei to capture electrons. This process is called recombination, during which the first neutral atoms took form. Once atoms become neutral, they only absorb photons of a discrete absorption spectrum. This allows most of the photons in the universe to travel unimpeded for billions of years. These photons are still detectable today in the cosmic microwave background radiation.
After Big Bang nucleosynthesis, no heavier elements could be created until the formation of the first stars. These stars fused heavier elements through stellar nucleosynthesis during their lives and through supernova nucleosynthesis as they died. The seeding of the interstellar medium by heavy elements eventually allowed the formation of terrestrial planets like the Earth.
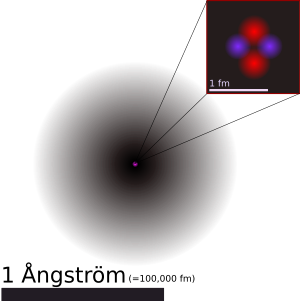
Size comparisons
Template:Trivia Various analogies have been used to demonstrate the minuteness of the atom:
- A human hair is about 1 million carbon atoms wide.
- A single drop of water contains about 2 sextillion atoms of oxygen (2 followed by 21 zeros, 2×10x21st and twice as many hydrogen atoms. ISBN 0-13-054091-9
}} Science textbook, Page 32: "There are 2,000,000,000,000,000,000,000 (that's 2 sextillion) atoms of oxygen in one drop of water—and twice as many atoms of hydrogen."
- An HIV virion is the width of 800 carbon atoms and contains about 100 million atoms total. An E. coli bacterium contains perhaps 100 billion atoms, and a typical human cell roughly 100 trillion atoms.
- A speck of dust might contain 3x10Template:Smsup (3 trillion) atoms.
- The number of atoms in 12 grams of charcoal (about 6 x 10x 23rd) is more than 1,400,000 times the age of the universe in seconds.
References
- Kenneth S. Krane, Introductory Nuclear Physics (1987)
- Atomic and cosmic model of ferman (1975. [3]
External links
- Atoms for Students
- Atomic sizes
- How Atoms Work
- Wikibooks FHSST Physics Atom:The Atom
- Wikibooks Atomic structure
- Science aid - atomic structure A guide to the atom for teens.
for more see: [4] File:Example.jpg