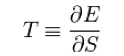Temperature
Etymology
Latin temperatura mixture, moderation, from temperatus, past participle of temperare
- Date: 1533
Definitions
- 1 archaic a : complexion 1
- b : temperament 3b
- b : the degree of heat that is natural to the body of a living being
- c : abnormally high body heat <running a temperature>
- b : mood <testing the temperature of voters>
Description
Temperature is a thermodynamic quantity that is related to the average energy of motion, or kinetic energy, of particles in matter.
Historically, two equivalent concepts of temperature have developed, the thermodynamic description and a microscopic explanation based on statistical physics. Since thermodynamics deals entirely with macroscopic measurements, the thermodynamic definition of temperature, first stated by Lord Kelvin, is stated entirely in empirical, measurable variables. Statistical physics provides a deeper understanding of thermodynamics by describing matter as a collection of a large number of particles, and derives thermodynamic (i.e. macroscopic) parameters as statistical averages of the microscopic parameters of the particles.
In statistical physics, it is shown that the thermodynamic definition of temperature can be interpreted as a measure of the average energy in each degree of freedom of the particles in the thermodynamic system. Because its temperature is seen as a statistical property, a system must contain a large number of particles for temperature to have a useful meaning. For a solid, this energy is found primarily in the vibrations of its atoms about their equilibrium positions. In an ideal monatomic gas, energy is found in the translational motions of the particles; with molecular gases, vibrational and rotational motions also provide thermodynamic degrees of freedom.
Temperature is a physical property that underlies the common notions of hot and cold. Something that feels hotter generally has a higher temperature, though temperature is not a direct measurement of heat. Temperature is one of the principal parameters of thermodynamics. If no net heat flow occurs between two objects, the objects have the same temperature; otherwise, heat flows from the object with the higher temperature to the object with the lower one. This is a consequence of the laws of thermodynamics.
Temperature is measured with thermometers that may be calibrated to a variety of temperature scales. In most of the world (except for Belize, Myanmar, Liberia and the United States), the Celsius scale is used for most temperature measuring purposes. The entire scientific world (these countries included) measures temperature using the Celsius scale and thermodynamic temperature using the Kelvin scale, which is just the Celsius scale shifted downwards so that 0 K[1]= −273.15 °C, or absolute zero. Many engineering fields in the U.S., notably high-tech and US federal specifications (civil and military), also use the Kelvin and Celsius scales. Other engineering fields in the U.S. also rely upon the Rankine scale (a shifted Fahrenheit scale) when working in thermodynamic-related disciplines such as combustion.[1]
For a system in thermal equilibrium at a constant volume, temperature is thermodynamically defined in terms of its energy (E) and entropy (S) as: