Difference between revisions of "Chain reaction"
(Created page with 'File:lighterstill.jpgright|frame *[http://en.wikipedia.org/wiki/20th_century 1902] ==Definitions== *1a : a series of events so related to ...') |
m (Text replacement - "http://" to "https://") |
||
| Line 1: | Line 1: | ||
[[File:lighterstill.jpg]][[File:Fission_chain.jpg|right|frame]] | [[File:lighterstill.jpg]][[File:Fission_chain.jpg|right|frame]] | ||
| − | *[ | + | *[https://en.wikipedia.org/wiki/20th_century 1902] |
==Definitions== | ==Definitions== | ||
*1a : a [[series]] of [[events]] so related to each other that each one [[initiates]] the next | *1a : a [[series]] of [[events]] so related to each other that each one [[initiates]] the next | ||
| Line 9: | Line 9: | ||
A '''chain reaction''' is a [[sequence]] of [[reactions]] where a reactive [[product]] or by-product causes additional reactions to take place. In a chain reaction, positive [[feedback]] leads to a self-amplifying chain of [[events]]. | A '''chain reaction''' is a [[sequence]] of [[reactions]] where a reactive [[product]] or by-product causes additional reactions to take place. In a chain reaction, positive [[feedback]] leads to a self-amplifying chain of [[events]]. | ||
| − | Chain reactions are one way in which [[systems]] which are in thermodynamic non-equilibrium can release [[energy]] or increase [ | + | Chain reactions are one way in which [[systems]] which are in thermodynamic non-equilibrium can release [[energy]] or increase [https://en.wikipedia.org/wiki/Entropy entropy] in order to reach a state of higher entropy. For example, a system may not be able to reach a lower [[energy]] state by releasing energy into the [[environment]], because it is hindered in some way from taking the [[path]] that will result in the energy release. If a [[reaction]] results in a small energy release making way for more energy releases in an expanding [[chain]], then the system will typically collapse [[explosively]] until much or all of the stored [[energy]] has been released. |
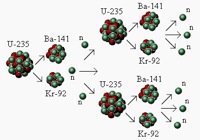
| − | A macrosopic [[metaphor]] for chain reactions is thus a snowball causing larger snowfall until finally an [ | + | A macrosopic [[metaphor]] for chain reactions is thus a snowball causing larger snowfall until finally an [https://en.wikipedia.org/wiki/Avalanche avalanche] results ("snowball effect"). This is a result of stored [[gravitational]] [[potential]] [[energy]] seeking a path of release over [[friction]]. Chemically, the equivalent to a snow avalanche is a spark causing a forest fire. In nuclear [[physics]], a single stray [https://en.wikipedia.org/wiki/Neutron neutron] can result in a [https://en.wikipedia.org/wiki/Prompt_critical prompt critical] event, which may be finally be energetic enough for a nuclear reactor meltdown or (in a bomb) a nuclear [[explosion]]. However, this reaction cannot be reversed.[https://en.wikipedia.org/wiki/Chain_reaction] |
[[Category: Physics]] | [[Category: Physics]] | ||
Latest revision as of 23:42, 12 December 2020
Definitions
- b : a number of events triggered by the same initial event
- 2: a self-sustaining chemical or nuclear reaction yielding energy or products that cause further reactions of the same kind
Description
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way in which systems which are in thermodynamic non-equilibrium can release energy or increase entropy in order to reach a state of higher entropy. For example, a system may not be able to reach a lower energy state by releasing energy into the environment, because it is hindered in some way from taking the path that will result in the energy release. If a reaction results in a small energy release making way for more energy releases in an expanding chain, then the system will typically collapse explosively until much or all of the stored energy has been released.
A macrosopic metaphor for chain reactions is thus a snowball causing larger snowfall until finally an avalanche results ("snowball effect"). This is a result of stored gravitational potential energy seeking a path of release over friction. Chemically, the equivalent to a snow avalanche is a spark causing a forest fire. In nuclear physics, a single stray neutron can result in a prompt critical event, which may be finally be energetic enough for a nuclear reactor meltdown or (in a bomb) a nuclear explosion. However, this reaction cannot be reversed.[1]